
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Basic Research
-
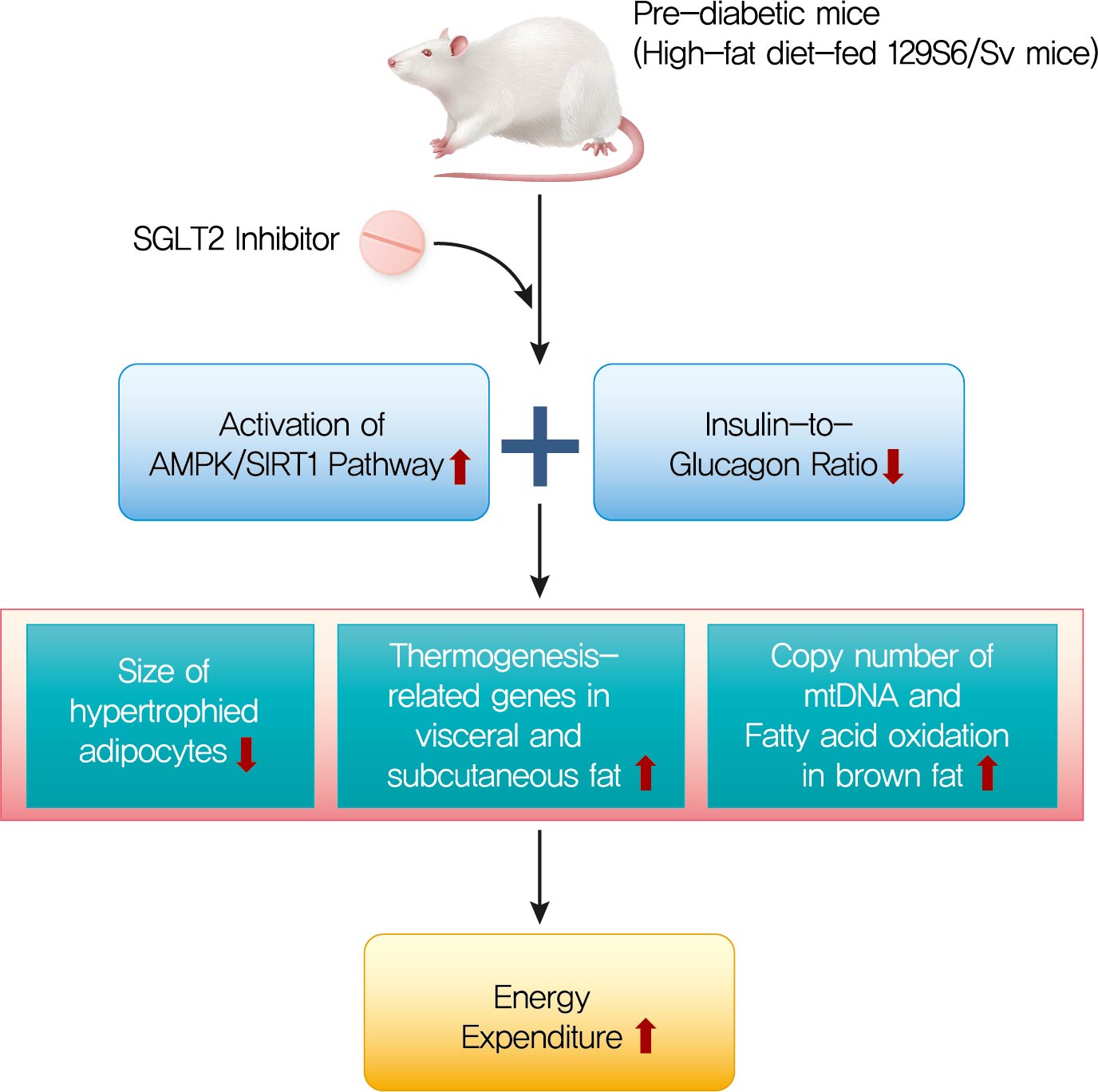
- Ipragliflozin, an SGLT2 Inhibitor, Ameliorates High-Fat Diet-Induced Metabolic Changes by Upregulating Energy Expenditure through Activation of the AMPK/ SIRT1 Pathway
- Ji-Yeon Lee, Minyoung Lee, Ji Young Lee, Jaehyun Bae, Eugene Shin, Yong-ho Lee, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha
- Diabetes Metab J. 2021;45(6):921-932. Published online February 22, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0187
- 8,507 View
- 410 Download
- 20 Web of Science
- 21 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
- Background
Sodium-glucose co-transporter 2 (SGLT2) inhibitors are a new class of antidiabetic drugs that exhibit multiple extraglycemic effects. However, there are conflicting results regarding the effects of SGLT2 inhibition on energy expenditure and thermogenesis. Therefore, we investigated the effect of ipragliflozin (a selective SGLT2 inhibitor) on energy metabolism.
Methods
Six-week-old male 129S6/Sv mice with a high propensity for adipose tissue browning were randomly assigned to three groups: normal chow control, 60% high-fat diet (HFD)-fed control, and 60% HFD-fed ipragliflozin-treated groups. The administration of diet and medication was continued for 16 weeks.
Results
The HFD-fed mice became obese and developed hepatic steatosis and adipose tissue hypertrophy, but their random glucose levels were within the normal ranges; these features are similar to the metabolic features of a prediabetic condition. Ipragliflozin treatment markedly attenuated HFD-induced hepatic steatosis and reduced the size of hypertrophied adipocytes to that of smaller adipocytes. In the ipragliflozin treatment group, uncoupling protein 1 (Ucp1) and other thermogenesis-related genes were significantly upregulated in the visceral and subcutaneous adipose tissue, and fatty acid oxidation was increased in the brown adipose tissue. These effects were associated with a significant reduction in the insulin-to-glucagon ratio and the activation of the AMP-activated protein kinase (AMPK)/sirtuin 1 (SIRT1) pathway in the liver and adipose tissue.
Conclusion
SGLT2 inhibition by ipragliflozin showed beneficial metabolic effects in 129S6/Sv mice with HFD-induced obesity that mimics prediabetic conditions. Our data suggest that SGLT2 inhibitors, through their upregulation of energy expenditure, may have therapeutic potential in prediabetic obesity. -
Citations
Citations to this article as recorded by- SGLT2 inhibitors and AMPK: The road to cellular housekeeping?
Nasser Safaie, Shahab Masoumi, Shaban Alizadeh, Pourya Mirzajanzadeh, Hamid Reza Nejabati, Mobasher Hajiabbasi, Vahid Alivirdiloo, Neda Chobdari Basmenji, Aysan Derakhshi Radvar, Ziba Majidi, Yousef Faridvand
Cell Biochemistry and Function.2024;[Epub] CrossRef - Mechanisms of SGLT2 Inhibitors in Heart Failure and Their Clinical Value
Yafei Xie, Yujie Wei, Dan Li, Jie Pu, Hong Ding, Xiaowei Zhang
Journal of Cardiovascular Pharmacology.2023; 81(1): 4. CrossRef - Current Treatment Options, Including Diet, Exercise, and Medications
Mazen Noureddin, Manal F. Abdelmalek
Clinics in Liver Disease.2023; 27(2): 397. CrossRef - SGLT2 Inhibitors and Kidney Diseases: A Clinical Perspective
Panagiotis Theofilis, Rigas G. Kalaitzidis
Current Medicinal Chemistry.2023; 30(23): 2595. CrossRef - Treatment of obesity-related diabetes: significance of thermogenic adipose tissue and targetable receptors
Ruping Pan, Jiadai Liu, Yong Chen
Frontiers in Pharmacology.2023;[Epub] CrossRef - Immunomodulatory Effects of SGLT2 Inhibitors—Targeting Inflammation and Oxidative Stress in Aging
Ema Schönberger, Vjera Mihaljević, Kristina Steiner, Sandra Šarić, Tomislav Kurevija, Ljiljana Trtica Majnarić, Ines Bilić Ćurčić, Silvija Canecki-Varžić
International Journal of Environmental Research and Public Health.2023; 20(17): 6671. CrossRef - SGLT‐2 inhibitors enhance the effect of metformin to ameliorate hormonal changes and inflammatory markers in a rat PCOS model
Manal Moustafa Mahmoud, Laila Ahmed Rashed, Somia Abdulatif Soliman, Safaa Mostafa Sayed, Omneya Kamel, Samaa Samir Kamar, Rania El Sayed Hussien
Physiological Reports.2023;[Epub] CrossRef - Resting energy expenditure based on equation estimation can predict renal outcomes in patients with type 2 diabetes mellitus and biopsy-proven diabetic kidney disease
Xiang Xiao, Shuming Ji, Junlin Zhang, Deying Kang, Fang Liu
Renal Failure.2023;[Epub] CrossRef - Sodium-glucose Cotransporter 2 Inhibitors and Pathological Myocardial
Hypertrophy
Zhicheng Gao, Jiaqi Bao, Yilan Hu, Junjie Tu, Lifang Ye, Lihong Wang
Current Drug Targets.2023; 24(13): 1009. CrossRef - SIRT1 mediates the inhibitory effect of Dapagliflozin on EndMT by inhibiting the acetylation of endothelium Notch1
Weijie Wang, Yilan Li, Yanxiu Zhang, Tao Ye, Kui Wang, Shuijie Li, Yao Zhang
Cardiovascular Diabetology.2023;[Epub] CrossRef - Direct cardio-protection of Dapagliflozin against obesity-related cardiomyopathy via NHE1/MAPK signaling
Ke Lin, Na Yang, Wu Luo, Jin-fu Qian, Wei-wei Zhu, Shi-ju Ye, Chen-xin Yuan, Di-yun Xu, Guang Liang, Wei-jian Huang, Pei-ren Shan
Acta Pharmacologica Sinica.2022; 43(10): 2624. CrossRef - Pleiotropic effects of SGLT2 inhibitors and heart failure outcomes
Panagiotis Theofilis, Marios Sagris, Evangelos Oikonomou, Alexios S. Antonopoulos, Gerasimos Siasos, Kostas Tsioufis, Dimitris Tousoulis
Diabetes Research and Clinical Practice.2022; 188: 109927. CrossRef - Role of Sodium-Glucose Co-Transporter 2 Inhibitors in the Regulation of Inflammatory Processes in Animal Models
Sandra Feijóo-Bandín, Alana Aragón-Herrera, Manuel Otero-Santiago, Laura Anido-Varela, Sandra Moraña-Fernández, Estefanía Tarazón, Esther Roselló-Lletí, Manuel Portolés, Oreste Gualillo, José Ramón González-Juanatey, Francisca Lago
International Journal of Molecular Sciences.2022; 23(10): 5634. CrossRef - Potential molecular mechanism of action of sodium-glucose co-transporter 2 inhibitors in the prevention and management of diabetic retinopathy
Lia Meuthia Zaini, Arief S Kartasasmita, Tjahjono D Gondhowiardjo, Maimun Syukri, Ronny Lesmana
Expert Review of Ophthalmology.2022; 17(3): 199. CrossRef - New insights and advances of sodium-glucose cotransporter 2 inhibitors in heart failure
Juexing Li, Lei Zhou, Hui Gong
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef - Critical Reanalysis of the Mechanisms Underlying the Cardiorenal Benefits of SGLT2 Inhibitors and Reaffirmation of the Nutrient Deprivation Signaling/Autophagy Hypothesis
Milton Packer
Circulation.2022; 146(18): 1383. CrossRef - Nutraceutical activation of Sirt1: a review
James J DiNicolantonio, Mark F McCarty, James H O'Keefe
Open Heart.2022; 9(2): e002171. CrossRef - Dapagliflozin Restores Impaired Autophagy and Suppresses Inflammation in High Glucose-Treated HK-2 Cells
Jing Xu, Munehiro Kitada, Yoshio Ogura, Haijie Liu, Daisuke Koya
Cells.2021; 10(6): 1457. CrossRef - Could Sodium/Glucose Co-Transporter-2 Inhibitors Have Antiarrhythmic Potential in Atrial Fibrillation? Literature Review and Future Considerations
Dimitrios A. Vrachatis, Konstantinos A. Papathanasiou, Konstantinos E. Iliodromitis, Sotiria G. Giotaki, Charalampos Kossyvakis, Konstantinos Raisakis, Andreas Kaoukis, Vaia Lambadiari, Dimitrios Avramides, Bernhard Reimers, Giulio G. Stefanini, Michael C
Drugs.2021; 81(12): 1381. CrossRef - Differential Pathophysiological Mechanisms in Heart Failure With a Reduced or Preserved Ejection Fraction in Diabetes
Milton Packer
JACC: Heart Failure.2021; 9(8): 535. CrossRef - Ketone bodies: from enemy to friend and guardian angel
Hubert Kolb, Kerstin Kempf, Martin Röhling, Martina Lenzen-Schulte, Nanette C. Schloot, Stephan Martin
BMC Medicine.2021;[Epub] CrossRef
- SGLT2 inhibitors and AMPK: The road to cellular housekeeping?
- Balsamic Vinegar Improves High Fat-Induced Beta Cell Dysfunction via Beta Cell ABCA1
- Hannah Seok, Ji Young Lee, Eun Mi Park, Se Eun Park, Jae Hyuk Lee, Seungtaek Lim, Byung-Wan Lee, Eun Seok Kang, Hyun Chul Lee, Bong Soo Cha
- Diabetes Metab J. 2012;36(4):275-279. Published online August 20, 2012
- DOI: https://doi.org/10.4093/dmj.2012.36.4.275
- 4,700 View
- 50 Download
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background The aim of this study was to investigate the effects of balsamic vinegar on β-cell dysfunction.
Methods In this study, 28-week-old Otsuka Long-Evans Tokushima Fatty (OLETF) rats were fed a normal chow diet or a high-fat diet (HFD) and were provided with tap water or dilute balsamic vinegar for 4 weeks. Oral glucose tolerance tests and histopathological analyses were performed thereafter.
Results In rats fed both the both chow diet and the HFD, the rats given balsamic vinegar showed increased insulin staining in islets compared with tap water administered rats. Balsamic vinegar administration also increased β-cell ATP-binding cassette transporter subfamily A member 1 (ABCA1) expression in islets and decreased cholesterol levels.
Conclusion These findings provide the first evidence for an anti-diabetic effect of balsamic vinegar through improvement of β-cell function via increasing β-cell ABCA1 expression.
-
Citations
Citations to this article as recorded by- The herbal extract ALS-L1023 from Melissa officinalis reduces weight gain, elevated glucose levels and β-cell loss in Otsuka Long-Evans Tokushima fatty rats
Yujin Shin, Dongju Lee, Jiwon Ahn, Mijeong Lee, Soon Shik Shin, Michung Yoon
Journal of Ethnopharmacology.2021; 264: 113360. CrossRef - The Effect of Balsamic Vinegar Dressing on Protein and Carbohydrate Digestibility is Dependent on the Food Matrix
Eleonora Urbinati, Mattia Di Nunzio, Gianfranco Picone, Elena Chiarello, Alessandra Bordoni, Francesco Capozzi
Foods.2021; 10(2): 411. CrossRef - Safety and side effects of apple vinegar intake and its effect on metabolic parameters and body weight: a systematic review
Tine Louise Launholt, Christina Blanner Kristiansen, Peter Hjorth
European Journal of Nutrition.2020; 59(6): 2273. CrossRef - Nypa fruticans Wurmb. Vinegar’s Aqueous Extract Stimulates Insulin Secretion and Exerts Hepatoprotective Effect on STZ-Induced Diabetic Rats
Nor Yusoff, Vuanghao Lim, Bassel Al-Hindi, Khairul Abdul Razak, Tri Widyawati, Dwi Anggraini, Mariam Ahmad, Mohd Asmawi
Nutrients.2017; 9(9): 925. CrossRef - Cholesterol in Pancreatic β-Cell Death and Dysfunction
Rajib Paul, Amarendranath Choudhury, Sabanum Choudhury, Muhammed K. Mazumder, Anupom Borah
Pancreas.2016; 45(3): 317. CrossRef - Chemical Characteristics and Immuno-Stimulatory Activity of Polysaccharides from Fermented Vinegars Manufactured with Different Raw Materials
Dong-Su Kim, Byung Serk Hurh, Kwang-Soon Shin
Journal of the Korean Society of Food Science and Nutrition.2015; 44(2): 191. CrossRef - Effect and mechanisms of action of vinegar on glucose metabolism, lipid profile, and body weight
Eleni I Petsiou, Panayota I Mitrou, Sotirios A Raptis, George D Dimitriadis
Nutrition Reviews.2014; 72(10): 651. CrossRef - Chemical Property and Macrophage Stimulating Activity of Polysaccharides isolated from Brown Rice and Persimmon Vinegars
Dong-Su Kim, Kwang-Soon Shin
The Korean Journal of Food And Nutrition.2014; 27(6): 1033. CrossRef - Vinegar ingestion at mealtime reduced fasting blood glucose concentrations in healthy adults at risk for type 2 diabetes
Carol S. Johnston, Samantha Quagliano, Serena White
Journal of Functional Foods.2013; 5(4): 2007. CrossRef
- The herbal extract ALS-L1023 from Melissa officinalis reduces weight gain, elevated glucose levels and β-cell loss in Otsuka Long-Evans Tokushima fatty rats
- Dietary Oleate Has Beneficial Effects on Every Step of Non-Alcoholic Fatty Liver Disease Progression in a Methionine- and Choline-Deficient Diet-Fed Animal Model
- Ji Young Lee, Jae Hoon Moon, Jong Suk Park, Byung-Wan Lee, Eun Seok Kang, Chul Woo Ahn, Hyun Chul Lee, Bong Soo Cha
- Diabetes Metab J. 2011;35(5):489-496. Published online October 31, 2011
- DOI: https://doi.org/10.4093/dmj.2011.35.5.489
- 30,211 View
- 34 Download
- 17 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Non-alcoholic fatty liver disease (NAFLD) is increasingly recognized as a major cause of liver-related morbidity and mortality. The underlying mechanisms of disease progression remain poorly understood, and primary therapy of NAFLD is not yet established. We investigated the effects of dietary oleate on the development and progression of NAFLD in a methionine- and choline-deficient (MCD) diet-fed animal model.
Methods A total of 30 C57BL/6J mice were randomly divided into three groups (
n =10 in each group) and fed various experimental diets for four weeks: chow, MCD diet, or OMCD (MCD diet with oleate, 0.5 mg/g/day). Liver samples were examined for steatohepatitis and fibrosis parameters and associated genes.Results Additional dietary oleate dramatically reduced MCD diet-induced hepatic steatosis. Hepatic carbohydrate responsive element-binding protein was overexpressed in MCD diet-fed mice, and dietary oleate prevented this overexpression (
P <0.001). Dietary oleate partially prevented MCD diet-induced serum level increases in aspartate aminotransferase and alanine aminotransferase (P <0.001, respectively). The mRNA expressions of hepatic monocyte chemoattractant protein 1, tumor necrosis factor-α and matrix metalloproteinase-9 were increased in MCD diet-fed mice, and this overexpression of inflammatory molecules was prevented by dietary oleate (P <0.001). Hepatic pericellular fibrosis was observed in MCD diet-fed mice, and dietary oleate prevented this fibrosis. Altogether, dietary oleate prevented MCD diet-induced hepatic steatosis, inflammation and fibrosis.Conclusion Dietary oleate has beneficial effects in every step of NAFLD development and progression and could be a nutritional option for NAFLD prevention and treatment.
-
Citations
Citations to this article as recorded by- Bidirectional association between NAFLD and gallstone disease: a systematic review and meta-analysis of observational studies
Shengying Gu, Shanshan Hu, Shuowen Wang, Chendong Qi, Chenyang Shi, Guorong Fan
Expert Review of Gastroenterology & Hepatology.2023; 17(3): 283. CrossRef - The Effect of Bioactive Aliment Compounds and Micronutrients on Non-Alcoholic Fatty Liver Disease
Camelia Munteanu, Betty Schwartz
Antioxidants.2023; 12(4): 903. CrossRef - Single‐cell transcriptomics stratifies organoid models of metabolic dysfunction‐associated steatotic liver disease
Anja Hess, Stefan D Gentile, Amel Ben Saad, Raza‐Ur Rahman, Tim Habboub, Daniel S Pratt, Alan C Mullen
The EMBO Journal.2023;[Epub] CrossRef - Histopathological Examination of the Effects of Tocilizumab and Dexamethasone on the Liver in Rats of Oleic Acid induced Acute Lung Injury
Funda TERZİ, Hüseyin Serkan EROL
Balıkesır Health Sciences Journal.2022;[Epub] CrossRef - Identifying Lipid Metabolites Influenced by Oleic Acid Administration Using High-Performance Liquid Chromatography–Mass Spectrometry-Based Lipidomics
Chao Xu, Dan Song, Askild L. Holck, Youyou Zhou, Rong Liu
ACS Omega.2020; 5(20): 11314. CrossRef - Causative and Sanative dynamicity of ChREBP in Hepato-Metabolic disorders
P. Vineeth Daniel, Prosenjit Mondal
European Journal of Cell Biology.2020; 99(8): 151128. CrossRef - PPARδ attenuates hepatic steatosis through autophagy-mediated fatty acid oxidation
Lei Tong, Long Wang, Shuangshuang Yao, Lina Jin, Jian Yang, Yifei Zhang, Guang Ning, Zhiguo Zhang
Cell Death & Disease.2019;[Epub] CrossRef - Butyrate Protects Mice Against Methionine–Choline-Deficient Diet-Induced Non-alcoholic Steatohepatitis by Improving Gut Barrier Function, Attenuating Inflammation and Reducing Endotoxin Levels
Jianzhong Ye, Longxian Lv, Wenrui Wu, Yating Li, Ding Shi, Daiqiong Fang, Feifei Guo, Huiyong Jiang, Ren Yan, Wanchun Ye, Lanjuan Li
Frontiers in Microbiology.2018;[Epub] CrossRef - Olive oil combined with Lycium barbarum polysaccharides attenuates liver apoptosis and inflammation induced by carbon tetrachloride in rats
Yun-Yun Chiang, Jane C.-J. Chao
Journal of Functional Foods.2018; 48: 329. CrossRef - Dietary oleic acid regulates hepatic lipogenesis through a liver X receptor-dependent signaling
Simon Ducheix, Alexandra Montagner, Arnaud Polizzi, Frédéric Lasserre, Marion Régnier, Alice Marmugi, Fadila Benhamed, Justine Bertrand-Michel, Laila Mselli-Lakhal, Nicolas Loiseau, Pascal G. Martin, Jean-Marc Lobaccaro, Laurent Ferrier, Catherine Postic,
PLOS ONE.2017; 12(7): e0181393. CrossRef - Is hepatic lipogenesis fundamental for NAFLD/NASH? A focus on the nuclear receptor coactivator PGC-1β
Simon Ducheix, Maria Carmela Vegliante, Gaetano Villani, Nicola Napoli, Carlo Sabbà, Antonio Moschetta
Cellular and Molecular Life Sciences.2016; 73(20): 3809. CrossRef - Metformin alleviates hepatosteatosis by restoring SIRT1-mediated autophagy induction via an AMP-activated protein kinase-independent pathway
Young Mi Song, Yong-ho Lee, Ji-Won Kim, Dong-Sik Ham, Eun-Seok Kang, Bong Soo Cha, Hyun Chul Lee, Byung-Wan Lee
Autophagy.2015; 11(1): 46. CrossRef - Adipokines and proinflammatory cytokines, the key mediators in the pathogenesis of nonalcoholic fatty liver disease
Sanja Stojsavljević
World Journal of Gastroenterology.2014; 20(48): 18070. CrossRef - Microglial Cell Activation Increases Saturated and Decreases Monounsaturated Fatty Acid Content, but Both Lipid Species are Proinflammatory
Emily B. Button, Andrew S. Mitchell, Marcia M. Domingos, Jessica H.‐J. Chung, Ryan M. Bradley, Ashkan Hashemi, Phillip M. Marvyn, Ashley C. Patterson, Ken D. Stark, Joe Quadrilatero, Robin E. Duncan
Lipids.2014; 49(4): 305. CrossRef - Modeling progressive non-alcoholic fatty liver disease in the laboratory mouse
Jesse D. Riordan, Joseph H. Nadeau
Mammalian Genome.2014; 25(9-10): 473. CrossRef - Rapid chromatographic method to decipher distinct alterations in lipid classes in NAFLD/NASH
Stephan Laggai, Yvette Simon, Theo Ranssweiler, Alexandra K Kiemer, Sonja M Kessler
World Journal of Hepatology.2013; 5(10): 558. CrossRef - Dimethyl sulfoxide reduces hepatocellular lipid accumulation through autophagy induction
Young Mi Song, Sun-Ok Song, Yong-Keun Jung, Eun-Seok Kang, Bong Soo Cha, Hyun Chul Lee, Byung-Wan Lee
Autophagy.2012; 8(7): 1085. CrossRef
- Bidirectional association between NAFLD and gallstone disease: a systematic review and meta-analysis of observational studies

 KDA
KDA
 First
First Prev
Prev





